

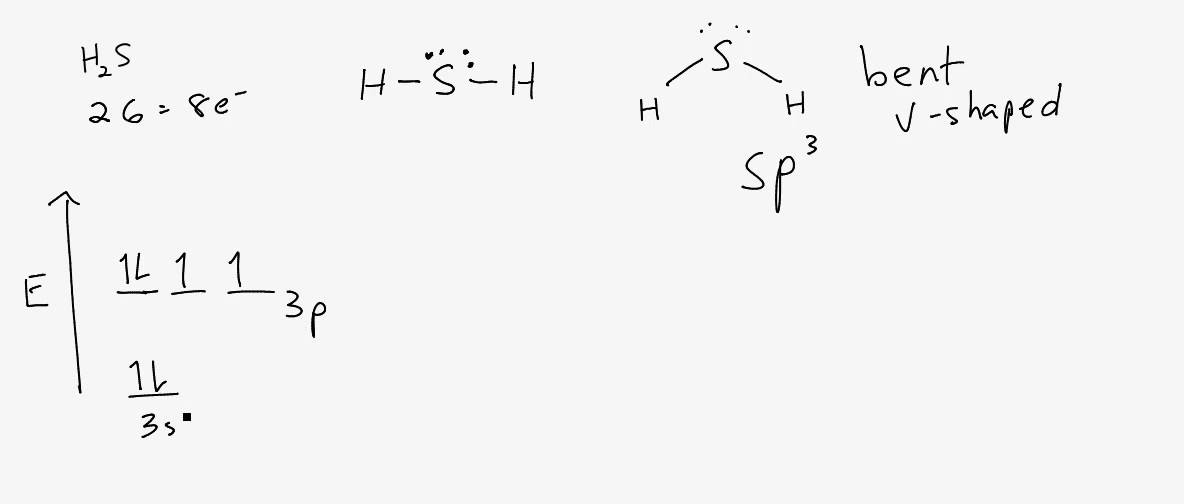
Last filled orbit, specific energy wave function must have eight electrons for satisfy the energy of stability of the particular canonical form, known as Octet rule which naturally can be seen in inactive inert gas molecules.įrom the Periodic table we can say Sulfur needs two more electrons to cover the 3p orbital where Hydrogen needs only one electron to stabilize its configuration like Helium, so both shares electron cloud and cover up their last filled shells. The angle between the bonding electron pair and non bonding electron pair is also decreased to adjust the new ‘V’ like bent structure. In the H2S lewis structure, the intermixing 3s, 3p orbital form sp3 hybridized orbital, so the covalent bond angle should be 109.5̊ but it is lowered to 92.1̊ by the steric repulsion between dense two non bonding electron pair of ‘S’.įor decreasing the bond angle (angle between the overlapping bonding orbital), the angle between the two nonbonding electron pairs increases for stabilizing the electron dot structure from the repulsion of dense lone electron pairs.

In the molecule only Sulfur atom has four electrons that not take part in bonding, so formal charge for the central atom Sulfur is = 0.Īs both the constituent atom has zero formal charge, this particular canonical form of H2S also has zero formal charge which makes the structure energetically stable one. H2S lewis acid structure shape H2s lewis structure formal chargeįormal charge of H2S lewis structure is zero, calculated to check stability of the canonical form with help of total outer shell electron, bonding electron cloud and unshared electron and assuming that the bonding electron are distributed equally. H2s lewis structure shapeĪccording to VBT theory molecular geometry and shape are two slightly different things if the central atom has unshared electrons, which can be clearly understood by the hybridization of orbital of the central atom of a covalent molecule.įrom the intermixing of orbital of the Sulfur atom in the H2S lewis structure generate hybrid orbital which is sp3, the geometry of the molecule should be tetrahedral type as for the AX2E2 where X stands for Hydrogen atom and E for electron lone pair.īut for the steric repulsion between lone pairs in the structure which Sulfur carries make the geometry disturbed from its original form and the angle between the bonding orbitals decreases forms a bent ‘V’ like shape. In spite of having 3d vacant orbital as a 3 rd period element canonical structure can’t form as the only one electron of Hydrogen atom is already involved in the covalent bonding. H2S lewis structure can’t form resonating structure as the ligand has no ‘d’ orbital for delocalization of electrons and also ligand atom has no unshared electron which can delocalize to the vacant 3d orbital of central atom Sulfur. H2S lewis structure H2s lewis structure resonance This H2S lewis structure follows the Octet rule with electron sharing and also has zero formal charge. Draw the skeletal of H2S lewis structure:Īs Hydrogen atom can’t coordinate with more than one atom so Sulfur become central atom and two Hydrogen atoms are written on the opposite sides. In modern Periodic table the Sulfur atom is in group 16 with electronic distribution in 3s and 3p orbital, that is 3s2 3p4 and Hydrogen atom contains only one electron its outer orbital as a group 1 element, so total eight loosely bonded valence electrons are there to form covalent bond.

With the atomic symbols of Sulfur (S) and Hydrogen (H), the H2S lewis structure shows the outer orbit electrons distribution, spreading around the particular atom and sharing electron cloud with neighbor atom in GeCl4 molecule. The article describes the H2S lewis structure with other properties it has which can be describe from the hybridization of the structure and the ‘d’ orbital that Sulfur carries.
H2S is a colorless gas, carries pungent smell like rotten egg, mainly used for producing Sulfuric acid, Sulfur, creating pesticides also used in nuclear power plant.


 0 kommentar(er)
0 kommentar(er)
